Knowing your Batteries - Part 2
by Larry Janke on 25 Nov 2009
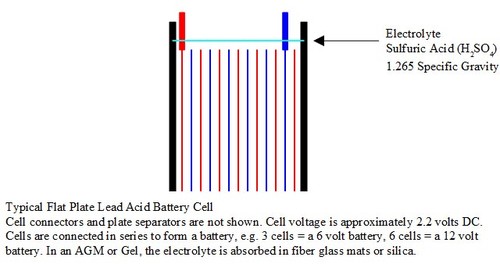
Typical flat plate lead acid battery SW
All sailing boats of any size use batteries, but do you know enough to choose the right one for your boat?
... to diagnose problems when they occur?
... to know that the 'expert' in that unknown port is telling you the right information?
Here www.semarine.com!Larry_Janke from SE Marine presents the second of a multi-part series on batteries that no longer-range sailor can afford not to read
Battery Design and Chemistry:
Three types of lead acid batteries are commonly used on boats, flooded, gel and absorbed glass mat (AGM), with flooded, because of superior longevity and capacity being predominant and preferred for cruising boats with intermittent charging and deep draw-down of the battery capacity on a regular basis. All of these depend upon lead acid chemistry, the difference being primarily the media in which the acid is contained and the composition of the grid material either lead antimony in the case of flooded types or lead calcium in the gels and AGMs. For power boats, and others that are not away from a charging source for long periods, the solid media batteries may be attractive because of their no maintenance feature. You will, however, pay a premium price for this convenience and cycle life will be less. AGM’s, however, make excellent engine cranking, windlass and thruster batteries.
Battery Sizes:
Battery sizes are defined by an industry organization, Battery Council International or BCI
Common BCI sizes are Group 24, Group 27, Group 31, 4D, and 8D etc. These designations refer only to case sizes and do not tell us much about the battery’s capacity or suitability for a particular application
Battery Ratings, What do they mean?
Amp Hour rating; The most common measure for batteries used in marine house banks is the amp hour rating, usually the 20 Hr rate although different hourly rates are used for other applications. The 20 hour rate is defined as the number of amps which may be taken from a battery over a period of 20 hours. For example; a 100 amp hour battery can de discharged at a rate of 5 amps for 20 hours. That does not mean that the same battery can be discharged at 10 amps for 10 hours. Battery capacity goes down proportionate to the amount of load placed on the battery. At a 10 amp draw the same battery would last approximately 8.5 hours.
Cranking Amps; Starting batteries are rated in cranking amps, Cold Cranking Amps (CCA or Marine Cranking Amps (MCA). Cold Cranking Amps is a rating used in the battery industry to define a battery's ability to start an engine in cold temperatures. The rating is the number of amps a new, fully charged battery can deliver at 0° F (-18° C)for 30 seconds, while maintaining a voltage of at least 7.2 volts, for a 12 volt battery (1.2 volts per cell). The higher the CCA rating, the greater the starting power of the battery
Marine Cranking Amps: is defined as the discharge load in amperes which a new, fully charged battery at 32 ° F (0°C), can continuously deliver for 30 seconds and maintain a terminal voltage equal or greater than 7.2 volts for a 12 volt battery (1.2 volts per cell).
Reserve Capacity: Is defined as the number of minutes a battery at 80 degrees F can be discharged at 25 amps and maintain a voltage of 10.5 volts for a 12 volt battery (1.75 volts per cell).
CCA MCA and reserve capacity have no application in house battery bank design. The higher the CCA for a given case size the more poorly a battery will cycle. Many high CCA batteries will fail completely in as few as 50 cycles if discharged to 50%. In selecting a starting battery of a given case size, more CCA or MCA is not necessarily better. Usually better longevity and service will be obtained from a battery some where between the highest and lowest available CCA. For example if you have a choice of 3 starting batteries of the same group rated at 600, 750, and 1000 amp hours the battery rated at 750 will probably give the best service.
Battery Types, a brief discussion of the pros and cons:
From the boater’s perspective, the type and usage of the vessel will, to a large part. Determine the best choice of battery types. There are two principal, and very different from an engineering point of view, applications for batteries in boats, engine starting and accessory or house power. Engine starting batteries, also called cranking batteries, and those that supply power to all of the many loads appliances and equipment present on modern vessels, navigation and communication electronics, lighting, refrigeration, entertainment, to name a few, generally called 'house batteries'.
Starting Batteries:
Starting batteries have the easier job; they are called upon to deliver large amounts of current (amps) for short periods of time to start an engine. These batteries work best if they have many thin plates and separators which allow rapid chemical reaction over a large plate area, (battery capacity is determined by the area of the plates), Starters, assuming normal engine start loads and times, give up very little of the power actually stored in them. For example, a starter rated at100 amp hours at the 20 hr rate if discharged at 400 amps for 5 seconds to start an engine will have given up about 0.56 amp hours or one half of one percent of capacity.
1 hour = 3600 seconds or 0.00028 hours
(400 amps x 5 seconds) = (2000 amp seconds x 0.00028 hrs.) = 0.56 amp hrs
That is why starting batteries very quickly become fully recharged after starting.
House Batteries:
House batteries on the other hand are the draft horses of the battery world, they do the hard part. House batteries are generally discharged at a much lower rate over long periods of time without the benefit of a charging source. The batteries are required to be cycle deeply, that is give up a large percentage of their stored power before recharging , hence the term 'deep cycle'. A deep cycle battery must be designed and constructed to withstand the stress of repeated discharge and recharge cycles.
The rule of thumb in the battery business is that batteries should not be discharged more than 50% of capacity, less is more (see cycle life curves on page 8). Although many batteries are advertised as 'deep cycle' or 'marine', the majority are, in reality, automotive starting batteries with rope handles and a label. The author is personally aware of a battery distributor who ordered a truckload of deep cycle batteries from a major manufacturer, upon arrival the batteries were labeled starting batteries and determined to be so. Upon complaint, the manufacturer sent a box of deep cycle labels. House batteries need thick heavy plates to survive deep cycle service.
Flooded batteries have the plates submerged in a solution of Sulfuric acid, Specific Gravity 1.265 at 78° F. which is approximately 34% acid and 66% water. Flooded battery development dates back to the discovery by Allesandro Volta, in 1800, that different substances submerged in a solution would create an electric current. After that, other scientist continued to make discoveries that lead to the invention of the first practical lead acid storage battery in 1860 by a Frenchman, Gaston Planté.
Improvements in manufacturing and materials have lead to the batteries we take for granted today. Flooded battery positive plates are constructed of lead grids upon which lead dioxide (PBO2) is pasted. Negative plates are constructed of sponge lead for maximum surface area. The grids are usually an alloy of lead and antimony in varying percentages depending upon the manufacturer.
The thicker and heavier plates use an antimony percentage in the neighborhood of 4.5% whereas manufacturers using thinner plates use higher percentages to add strength. The more antimony however the more prone the battery is to self discharge, (flooded lead antimony batteries self discharge at a rate of 0.5 to 1% or more per day depending upon the percentage of antimony present in the alloy).
Higher antimony percentages also increase gassing. One other effect of the presence of antimony is a so called antimony poisoning. As the battery cycles, antimony migrates from the positive to the negative plates, coating the latter and eventually blocking reaction with the lead. This phenomenon ultimately limits the theoretical life of a lead antimony battery to 20-25 years; however with the exception of the Rolls 5000 series batteries those figures are far more theoretical than actual.
Flooded lead acid starting batteries are usually sealed and generally have grids composed of lead and calcium. These batteries are not suitable for deep cycle house applications. Lead calcium grids will be discussed more thoroughly in the sections on AGM and Gel construction.
If you are a cruising sailor, flooded batteries are the preferred choice for deep cycle service where extended periods of discharge with out a charging source are interspersed between periodic charging. Flooded batteries require the greatest amount of maintenance but the higher quality products such as Rolls/Surrette deliver more cycle life by a substantial margin and in the long run are less expensive and worth the extra cost.
At the top of the list, Rolls/Surrette 4000 series will deliver 1350 50% discharge cycles which translates to 8-10 years of service, the Rolls/Surrette 5000 series batteries will deliver 3300 50% cycles and can last up to 20 years. If you are a weekend or occasional sailor and do not plan to keep your boat awhile, you may not want to put out the additional money for these high quality batteries and can probably get by with less expensive brands such as Trojan which will yield 50% discharge cycles in the 325 to 550 cycle range depending upon the model.
Most other flooded batteries do not meet the requirements for extended deep cycle service and should be avoided if longevity is desired or you are going outside the U.S. or Canada.
Power boats which have engines running and alternators or generators and alternating current powered chargers in operation generally impose less stringent requirements on house batteries than do sail boats, however if you anchor out for extended periods of time with infrequent recharge, the higher quality batteries may be a better value as they will withstand the cycling stresses better.
Absorbed Glass Mat (AGM) Batteries:
AGM batteries are lead acid batteries. The acid is absorbed into fiberglass mats rather than as a free liquid in the cell. The AGMs are recombinant batteries, that is, the oxygen and hydrogen produced during recharge recombine to water inside the cell.
They are known in the battery industry as Valve Regulated Lead Acid (VRLA) batteries. Although they are sealed, each cell has a valve which will open if excessive gassing occurs from overcharging or thermal runaway as discussed below. The grids are alloyed with calcium, rather than antimony as in flooded batteries. The presence of calcium retards gassing during recharge and also retards self discharge to about 1% per month. AGMs have the attractive features of being maintenance free, no hydrogen or oxygen is released during recharge and can be installed in any position, except upside down.
Although many claims are made by marketing departments for AGMs, they too have some limitations. The author has yet to see an AGM in a given case size which will deliver the same 20 hour capacity of a high quality deep cycle of the same case size. The graph below illustrates the discharge characteristics and power output of two 8Ds,(identical case sizes), one an AGM of advertised 255 amp hours at the 20 hour rate and the other a high quality flooded deep cycle of 275 tested amp hours at the 20 hour rate.
The top line on the graph is the flooded battery; the lower line is the AGM. The batteries were discharged at the 20 hour currents of 12.5 A (AGM) and 13.8 A (flooded) respectively. These current draw rates correspond to the 20 hour rates of the batteries, advertised in the case of the AGM and tested for the flooded battery. Actual power supplied by the batteries vs. elapsed time of discharge was electronically recorded and stored. The 'power' was directly measured and is a product of current and voltage. Power in watts = volts x amps. Since this is a constant current discharge, the power drop is a directly proportional measurement of voltage drop.
The AGM began discharge with a power output of 157 watts and reached full discharge in about 14 hours with an end power output of 131 watts. The flooded battery, because of its greater 20 hour capacity and consequent higher discharge rate, supplied an initial power rate of 181 watts and reached full discharge at 19 hours with an ending power output of 144 watts. It is interesting to note that shortly prior to the ending time for the flooded battery it was producing 151 watts, nearly as much as the AGM produced at the beginning of the discharge, (157 watts).
It is sometimes claimed that AGMs will maintain voltage better under load than a flooded battery. The curves on the above graph are quite parallel, evidencing comparable proportionate voltage maintenance at all stages of discharge. The important thing to be aware of however, is the area under the respective curves which represents the total power output of each battery.
As can be readily seen, the total power output in watts of the flooded 8D is substantially greater than the identical case size AGM. There are several reasons for this discrepancy, but the principal one would appear to be less plate area resulting in less overall capacity, since VRLA batteries must leave room in the case for the recharge gases to allow recombination and, as a result, plate area must be sacrificed.
Additionally, VRLA batteries are known as 'acid starved'. You cannot put as much electrolyte (acid) into the mats as in a free liquid. In the author’s experience, an AGM, of a given case size, will have an actual 20 hour rate amp hour of about 85% of an average flooded battery and only 70% of the capacity of the highest quality flooded batteries.
Another design feature of VRLA batteries is grid alloy. As mentioned above, the grids in these batteries are usually an alloy of lead and calcium. Although, some specialty batteries are a lead tin alloy, but these are not often used in the marine market, nor are they particularly well suited for deep cycle operation.
Lead calcium batteries, as recited above, have lower rates of gassing and self discharge, but again, we do not get anything for free. Lead calcium grids create calcium oxide during recharge cycles, this compound is both corrosive and accumulates, causing plate growth, eventual shorting and probably contributes to thermal runaway described below.
An important issue to be considered if selecting AGM batteries is the phenomenon of thermal runaway. Thermal runaway is defined as an uncontrolled temperature rise during the charging cycle accompanied by a rapid increase in charging current. This condition requires very careful attention to battery charger selection and charging parameter settings. The reasons for thermal runaway are not fully understood, but are thought to be caused by the design of the batteries themselves. AGM as stated in a preceding paragraph are acid starved.
It is thought that this condition interferes with oxygen migration to the negative plate during recharge causing an increase in temperature. It is probable that calcium oxide plate growth also contributes to this migration impedance. One researcher measured an increase in internal temperature to 210° F (99°C) with an increase of only 0.3 volts in a 48 volt system. For a further, more extensive discussion of thermal runaway see Jaworski and Hawkins.
Although it has been claimed that AGMs can be charged at very high rates without consequences such as heating, Jaworski’s results contradict this. It is probable that the fiberglass mats make an excellent insulator and internal temperatures are not registered on the outside of the battery case. It is also claimed that lower 'internal resistance' of AGMs allows more rapid charging, as a matter of fact, the actual internal resistance or more properly impedance of all lead acid batteries is very low.
The author has autopsied AGMs charged at high rates and found extensive damage in the form of warped plates, burned connector bars and other destruction. If traveling to geographical areas with high ambient temperatures, AGMs would probably not be the best choice.
AGMs are expensive, comparable or higher in cost to same case size high quality flooded batteries. They have all the limitations stated above and despite the claims made for high cycle life, the author has yet to see this substantiated. A best guess is that the higher quality AGM will yield at maybe 600-700 50% cycles.
So what are AGM’s good for? If you are unable or unwilling to perform the maintenance required of flooded batteries then AGMs may be a good house bank compromise if careful attention is paid to charging and allowance made for the decreased case size to case size capacity.
They make excellent engine starting, windlass and thruster batteries. For power boats that do not anchor out for long periods without charging and with carefully regulated recharge they are an excellent choice due to their no maintenance feature. It is important to remember that charging parameters are critical and capacity will be less than a quality flooded battery of the same case size.
Gel Batteries:
Gel batteries are similar to AGMs except that the electrolyte is mixed with silica to form a gel. They are also acid starved and have slightly less case size capacity than a comparable AGM and considerably less than flooded. Gel batteries are charge rate sensitive and, if over charged, will open the valves and release the hydrogen and oxygen which used to be water, drying out the plates and ruining the battery. In the author’s experience one bad recharge cycle can ruin a gel battery.
Gels do not seem to exhibit the thermal runaway phenomenon of AGMs. By way of history, about 30 years ago, somebody began vigorously marketing 'New Gel Batteries' with all kinds of claims as to their wonderful abilities. 'New Gel Batteries' were actually invented in 1928,(78 years ago) proving once again that scientists cannot change the laws of physics, only the marketing people can. There does not seem to be a good reason to use gel batteries in marine applications.
One other issue, which should be addressed, is because we cannot measure specific gravity in a VRLA battery, careful attention must be paid to accurate monitoring to avoid over discharge. Monitoring will be discussed in more detail later.
In summary, premium quality flooded lead acid batteries will provide much greater capacity and longevity than will AGM or gel type batteries. However, not every boat is an appropriate application for long lived flooded batteries. As discussed above, the batteries should be fitted to the application. When purchasing batteries, the consumer should ask the questions;
The following equation illustrates the chemical process taking place during charge and discharge cycles. During the discharge process, lead dioxide on the positive plate and lead on the negative plate react with sulfuric acid to form lead sulfate and water, releasing 2 electrons, during recharge the reverse occurs.
PbO2 + Pb + 2H2S04 = 2PbSO4 + 2H20
Charging Discharging
PbO2, lead dioxide, (positive plate)
Pb, lead, (negative plate)
PbSO4, lead sulfate, forms during discharge cycle
H2SO4, sulfuric acid, (electrolyte, specific gravity 1.265 at full charge)
H2O, battery electrolyte is approximately 34% acid and 66% water
Premium quality flooded lead acid batteries will provide much greater capacity and longevity than will AGM or gel type batteries. Charge cycle tests reveal that at 78F. a typical AGM will produce about 69% of the amp hr capacity of a quality flooded lead acid of the same case size and a gel a little less. At 32F AGM capacity will diminish to about 40% of capacity at 78F.
The explanation for these differences is primarily that a sealed, AGM or gel battery which is commonly referred to as Valve Regulated Lead Acid batteries, (VRLA) are designed to reabsorb the gas (hydrogen and oxygen) created during the charging cycle. To accomplish this, room must be left in the case for the gas; consequently, smaller plates are used. Since battery capacity in amp hrs is directly proportional to plate area, capacity declines. Cycle life is also affected. In an attempt to increase capacity more plates are used but that requires thinner plates and separators and cycle life suffers
A detailed analysis of the reasons for the cycle life difference is beyond the scope of this discussion, but includes, plate thickness, positive plate material density, grid alloy density, separator thickness and composition. Other limiting factors are inclusion or lack of encapsulation of individual plates and chemical or physical phenomena associated with the differences between antimony and. calcium alloy grids such as plate warpage, plate corrosion and formation of calcium oxides and hydroxides during the charge cycle.
The graph to the right illustrates the life cycles available at varying depth of discharge for various batteries
HISTORY
Some years ago, when boat electrical systems began to evolve from just engine starting and a few lights to the power consuming monsters of today, the requirement for readily available cycle capable batteries became apparent. At that time the garden variety golf cart was called in to service because it and in some cases telecom batteries were the only widely available battery with the required characteristics.
But the telcom batteries were usually used and irreplaceable and golf cart and larger L-16 size case had their limitations, principally in that it took too many of them to make up a battery bank of sufficient capacity to meet the needs of a large system.
For example, if we wanted to make up a bank with a capacity of say 800-1000amp hrs, with golf carts at about 200-225 amp hrs at the 20 hr rate we would need 8 at 12 volts and 24 cells to water.. With each cell we add to the system the probability of cell failure goes up (see cell failure probability curve.) If we jump up to L-16 size batteries (350 amp hrs at 20 hr rate,) we will still use 6 batteries for 12 volts with 18 cells to water.
Also, the number of connections goes up with every battery we add to the bank, 4 connections and 3 cables per battery, with each connection and inter-connecting cable creating a potential trouble spot. To insure proper charging and evenly distributed discharge stress it is important that each inter connecting cable be the same length and that the electron flow path from negative to positive is continuous throughout the entire bank, that is, that we come in from one end and go out the other.
I am sure in this diverse world of ours there is someone out there that actually enjoys maintaining batteries but I have yet to meet him, he is probably too busy repairing his bed of nails.
The most common design flaws that I see in battery banks besides too many cells are random length inter- connecting cables and improper current flow through the bank.
As this diagram illustrates, to assure proper charging, load and charge current conductors should be connected so there is an electron path through the entire bank, not taken off the end cells of the bank (red conductors). All inter-connecting cables should be the same length.
For more information about these subjects, go to Larry Janke's website, www.semarine.com , and don't forget to watch out on Sail-World Cruising for his next article on batteries, next week.
.......................
Letter from Reader:
Sender: William Seifert
Message: Rolls/Surette has changed the manufacturing of their golf cart batteries. The presently available GC batteries are basically no good. I have gone through three sets of 8 Rolls/Surette GC batteries in three years, and have changed to Trojan.
If you want to link to this article then please use this URL: www.sail-world.com/63700